TIM-3 Alzheimer’s Treatment: A Revolutionary Approach
TIM-3 Alzheimer’s treatment represents a groundbreaking approach that exploits the immune system to combat Alzheimer’s disease. Recent research highlights how inhibiting the TIM-3 gene can unleash microglia—brain immune cells—to target and eliminate toxic amyloid plaques that disrupt cognitive function. By blocking this inhibitory checkpoint molecule, scientists have observed promising improvements in memory and cognition in mouse models, paving the way for potential human therapies. This method not only responds to the genetic underpinnings linked to late-onset Alzheimer’s but also utilizes existing strategies from cancer treatments, offering hope in the battle against this degenerative condition. As Alzheimer’s disease research continues to evolve, TIM-3 emerges as a crucial player in restoring brain health and function.
The fight against Alzheimer’s disease is taking an innovative twist with the emergence of treatments centered around TIM-3, a checkpoint molecule historically associated with immune response regulation. By leveraging insights from immune system Alzheimer’s studies, researchers aim to enhance the brain’s ability to clear harmful plaques that contribute to cognitive decline. This therapeutic strategy seeks to boost the activity of microglia, the brain’s resident immune cells, ultimately restoring memory and overall brain health. Emerging therapies—particularly anti-TIM-3 therapies—have the potential to transform our understanding and treatment of late-onset Alzheimer’s, utilizing genetic insights to reshape therapeutic approaches. As scientists explore this avenue, the hope is that such interventions could dramatically change the landscape of Alzheimer’s treatment.
Understanding TIM-3 and Its Role in Alzheimer’s Disease
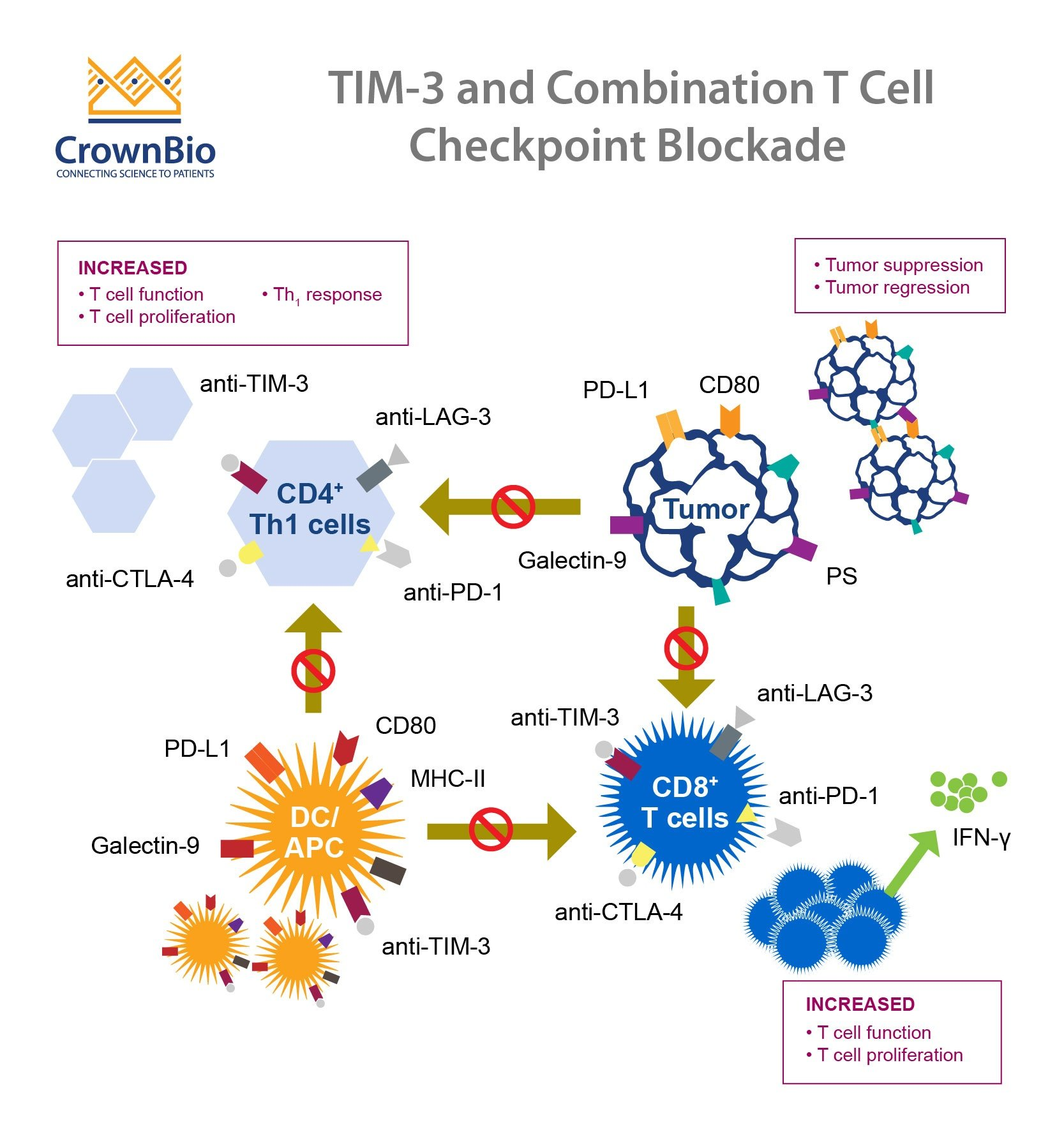
The TIM-3 gene has emerged as a critical player in Alzheimer’s disease research, primarily due to its association with late-onset Alzheimer’s. Genome-wide association studies have identified polymorphisms in the TIM-3 gene, specifically HAVCR2, which are prevalent in patients suffering from this neurodegenerative condition. In essence, TIM-3 acts as a checkpoint molecule within the immune system, inhibiting the activation of immune cells called microglia. As Alzheimer’s progresses and amyloid plaques accumulate, the TIM-3 protein expression increases on microglia, effectively preventing these immune cells from clearing out harmful plaques.
This inhibition of microglial activity poses a significant dilemma in Alzheimer’s pathology. While TIM-3 works to prevent the immune system from overreacting and causing further brain damage, its heightened expression in Alzheimer’s leads to a shrinkage in microglial responsiveness. Consequently, instead of attacking and clearing the amyloid beta plaques, these immune cells become homeostatic, leaving toxic materials in the brain, which continues to impair cognitive functions.
Innovative Therapy Strategies: Anti-TIM-3 for Alzheimer’s Treatment
Recent research suggests that targeting the TIM-3 receptor with anti-TIM-3 therapy could revolutionize our approach to Alzheimer’s disease treatment. By employing either anti-TIM-3 antibodies or small molecules capable of blocking TIM-3’s inhibitory effect, scientists aim to re-activate microglia so they can efficiently clear amyloid plaques. This kind of therapy harnesses an immune-response strategy that has shown promise in cancer treatments, as checkpoint inhibitors successfully unleash the immune system against tumors. If these treatment methods can be effectively adapted for Alzheimer’s, we could see significant advancements in cognitive preservation and recovery.
Ongoing preclinical trials indicate that deleting the TIM-3 gene in mice models leads to a notable decrease in plaque burden, restoring some degree of cognitive function. As researchers utilize mice with the human TIM-3 gene inserted to test the efficacy of anti-TIM-3 therapies, early results are promising. The hope is that repurposing existing anti-TIM-3 antibodies for Alzheimer’s treatment not only enhances plaque clearance but also circumvents the vascular issues associated with traditional anti-amyloid therapies, thus paving the way for more effective dementia interventions.
Developments in this therapeutic approach are significant, with several studies indicating that patients’ cognitive functions could improve markedly through well-designed anti-TIM-3 treatments. Nevertheless, clinical trials will be crucial to evaluate safety and effectiveness before these strategies can be implemented in human patients.
The Role of Microglia in Alzheimer’s Pathology
Microglia are often referred to as the immune cells of the brain, playing a pivotal role not only in disease defense but also in maintaining neural health through processes such as synaptic pruning. In the context of Alzheimer’s disease, these cells are essential for clearing away amyloid plaques; however, as age-related changes and the effects of the TIM-3 molecule take hold, microglia’s ability to perform these functions diminishes. When TIM-3 is overexpressed, it suppresses the phagocytic activity of microglia, which results in plaque accumulation and impaired neuronal function.
Understanding the dynamics of microglial activity is crucial for advancing Alzheimer’s research. As the balance between synaptic pruning and plaque removal shifts in favor of maintaining homeostasis, the detrimental consequences of plaque buildup become pronounced. This highlights the need for therapeutic interventions that can restore microglial functionality and re-enable the clearance of toxic substances in the brain, setting the stage for developing effective treatments targeting the TIM-3 pathway.
Future Research Directions in Alzheimer’s Disease Treatment
Moving forward, the research landscape regarding TIM-3 and Alzheimer’s disease is positioned to evolve dramatically. With emerging data supporting the link between TIM-3 gene polymorphisms and late-onset Alzheimer’s, future studies will likely explore further genetic insights into this relationship. By investigating how variations in the TIM-3 gene influence disease severity and onset, researchers hope to tailor both preventive and therapeutic strategies that can mitigate risk factors associated with late-onset Alzheimer’s.
Additionally, exploring the intricacies of immune response modulation through TIM-3 will open new avenues for treatment innovations. This includes detailed studies on how TIM-3 blockade can be effectively integrated into existing treatment protocols, as well as discovering complementary treatments that target other pathways influencing neuroinflammation and amyloid deposition. These collective efforts aim to provide a multi-faceted approach to Alzheimer’s disease, enhancing the quality of life for millions affected by this challenging condition.
The Implications of TIM-3 Research in Alzheimer’s Disease
The implications of TIM-3 research extend beyond immediate treatment strategies for Alzheimer’s, offering a broader understanding of the immune system’s role in neurodegenerative diseases. As we deepen our comprehension of how TIM-3 functions within the context of Alzheimer’s, new paradigms for understanding the interplay between immunity and neurodegeneration will emerge. This knowledge can lead to preventive initiatives aimed at lowering risk or delaying onset in at-risk populations.
Furthermore, the potential development of drugs that can modify TIM-3 activity presents opportunities not only for Alzheimer’s therapy but potentially for a spectrum of neurodegenerative disorders where similar immune mechanisms may play a role. As researchers explore avenues to manipulate TIM-3 expression and its effects, the broader neurological research community stands to gain insights that could transcend Alzheimer’s treatment, offering a more holistic view of neuroimmune interactions in various pathologies.
How TIM-3 Gene Polymorphisms Affect Alzheimer’s Risk
Recent studies focusing on the TIM-3 gene reveal a concerning correlation between specific polymorphisms and increased susceptibility to Alzheimer’s disease. These genetic variations affect how TIM-3 is expressed on microglia, with higher levels observed in patients with Alzheimer’s, suggesting an inherited risk for developing the disease. Understanding these genetic markers not only provides insights into disease pathology but also opens up avenues for genetic screening and targeted interventions.
By elucidating the mechanisms by which TIM-3 gene polymorphisms contribute to risk, researchers can better identify individuals who may benefit from early preventative strategies or clinical trials for emerging therapies. This genetic perspective reinforces the importance of personalized medicine in the fight against Alzheimer’s, where tailored therapies based on an individual’s genetic makeup could ultimately improve outcomes and enhance treatment efficacy.
Exploring the Therapeutic Potential of Microglia Activation
Enhancing microglia activation represents a promising target in the quest for effective Alzheimer’s treatments. Research has indicated that restoring the function of microglia could significantly impact the progression of the disease, particularly in clearing amyloid beta plaques from the brain. This assumes even greater importance as scientific understanding expands regarding how microglia contribute to both neuroprotection and neurotoxicity depending on their activation state.
The therapeutic modulation of microglial activation, such as through TIM-3 inhibition, exemplifies how scientists are attempting to flip the switch on these immune cells. By facilitating a transition from a homeostatic to an active state, researchers aim to restore the microglia’s natural ability to clear pathological deposits, which could lead to a substantial reduction in Alzheimer’s progression and an improvement in cognitive functions.
Translating TIM-3 Research into Clinical Interventions
As TIM-3’s role in Alzheimer’s treatment becomes clearer, the translation of findings from basic research into clinical trials is imperative. Researchers are keen to initiate trials using anti-TIM-3 therapies, leveraging existing antibodies that have successfully targeted TIM-3 in other conditions like cancer. These trials aim to assess not only the safety and effectiveness of these treatments in humans but also their strategic application in halting or reversing the pathological changes associated with Alzheimer’s.
The move toward clinical applications emphasizes the need for robust study designs that will evaluate the impact of TIM-3 modulation on cognitive function and memory retention in patients. By bridging the gap between experimental models and human subjects, researchers anticipate a future where TIM-3 therapies not only offer symptomatic relief but might also slow disease progression, ushering in a new era of Alzheimer’s disease management.
Frequently Asked Questions
What is TIM-3 in the context of Alzheimer’s treatment?
TIM-3, short for T-cell immunoglobulin and mucin-domain containing-3, is an inhibitory checkpoint molecule that regulates immune responses. In Alzheimer’s disease research, TIM-3 is linked to the pathology of the disease, as it inhibits microglia, the brain’s immune cells, from clearing amyloid plaques. Targeting TIM-3 may allow microglia to effectively attack these plaques, potentially leading to new treatments.
How does TIM-3 affect microglia in Alzheimer’s disease?
In Alzheimer’s disease, TIM-3 is highly expressed on microglia, preventing them from clearing accumulated amyloid beta plaques in the brain. This inhibition leads to further plaque accumulation and exacerbates cognitive decline. By targeting TIM-3, researchers hope to restore the ability of microglia to clear these harmful plaques.
What role does TIM-3 play in the immune system and Alzheimer’s disease pathology?
TIM-3 is a checkpoint molecule that normally serves to inhibit excessive immune responses. However, in the context of Alzheimer’s disease, upregulated TIM-3 on microglia prevents these cells from processing and removing amyloid plaques, thus contributing to disease progression. Research focusing on anti-TIM-3 therapies aims to unleash the potential of microglia to mitigate plaque-related damage.
What findings have emerged from recent studies on TIM-3 and Alzheimer’s treatment?
Recent studies have shown that deleting TIM-3 in mouse models of Alzheimer’s disease enables microglia to clear amyloid plaques more effectively and improves cognitive functions. This suggests that anti-TIM-3 therapies could enhance the immune system’s capability to combat Alzheimer’s by facilitating plaque clearance.
What potential therapies targeting TIM-3 exist for Alzheimer’s treatment?
Potential therapies for Alzheimer’s treatment targeting TIM-3 include the use of anti-TIM-3 antibodies or small molecules that inhibit TIM-3’s function. These treatments aim to enhance the activity of microglia, allowing them to clear amyloid plaques and possibly reverse cognitive decline associated with Alzheimer’s disease.
Can TIM-3 research lead to advancements in Alzheimer’s disease therapies?
Yes, research on TIM-3 could significantly impact Alzheimer’s disease therapies. By understanding how TIM-3 influences microglia’s ability to process amyloid plaques, scientists are developing strategies to counteract this inhibition, which could lead to effective new treatments for Alzheimer’s disease.
What challenges exist in translating TIM-3 research into Alzheimer’s treatment?
Translating TIM-3 research into Alzheimer’s treatment presents challenges such as ensuring therapeutic agents effectively cross the blood-brain barrier and selectively target TIM-3 on microglia without causing unwanted immune reactions. Ongoing research aims to overcome these hurdles and transition from successful animal studies to human clinical trials.
How might TIM-3 gene polymorphisms impact Alzheimer’s disease risk?
Polymorphisms in the TIM-3 gene have been identified as potential genetic risk factors for late-onset Alzheimer’s disease. These genetic variations may influence the expression of TIM-3 on microglia, thus affecting their ability to clear amyloid plaques and potentially increasing an individual’s risk for developing Alzheimer’s.
What are the long-term goals of TIM-3 related research in Alzheimer’s disease?
The long-term goals of TIM-3 related research in Alzheimer’s disease include developing viable therapies that can halt plaque accumulation, improve cognitive function, and ultimately change the course of the disease. Researchers aspire to bring anti-TIM-3 therapies from the lab to clinical practice to benefit patients.
What types of studies are currently exploring TIM-3’s role in Alzheimer’s disease?
Current studies exploring TIM-3’s role in Alzheimer’s disease predominantly focus on animal models, particularly those engineered to express human TIM-3. These studies assess the efficacy of anti-TIM-3 therapies in improving plaque clearance and cognitive functions, paving the way for future human trials.
| Key Point | Details |
|---|---|
| Introduction to TIM-3 Alzheimer’s Treatment | A study suggests that TIM-3, an immune checkpoint molecule linked to Alzheimer’s, may enable microglia to attack and clear beta-amyloid plaques. |
| Role of Microglia | Microglia act as immune cells in the brain that prune unnecessary synapses but become homeostatic with TIM-3, preventing clearance of plaques. |
| Research Findings | Deleting TIM-3 expression in mice improved memory and cognitive functions by enhancing plaque clearance. |
| Mechanism of Action | TIM-3 inhibits microglial activity, preventing them from attacking harmful plaques accumulating in the brain, which damages cognitive function. |
| Potential Human Therapy | Proposed treatments include anti-TIM-3 antibodies or small molecules to block TIM-3 actions in human Alzheimer’s patients. |
| Research Development Timeline | The study progressed over five years with extensive collaboration among researchers. |
Summary
TIM-3 Alzheimer’s treatment represents a promising new avenue in combating Alzheimer’s disease by targeting a crucial checkpoint molecule that prevents microglial cells from clearing harmful plaques in the brain. This innovative approach has shown potential in preclinical studies where TIM-3 was deleted, leading to improved cognitive functions in mice. The research opens the possibility of developing effective therapies utilizing anti-TIM-3 antibodies to enhance the brain’s immune response. As trials progress, this strategy could revolutionize how Alzheimer’s disease is treated, providing hope for those affected by this debilitating condition.